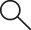
Organisation
The CoBi has its local headquarters in Dresden, where both the Clinical Trials Unit research department of DKMS Group GmbH and the sample manager are located.
The work to establish the CoBi began in the 3rd quarter of 2015 with the coordinative planning for the scientific and technical design of the Biobank. The CoBi has been approved by the Ethics Committee at the Technical University of Dresden, which marked the start of the further involvement of the collaboration partners.
Management of the CoBi
The administrative and coordinative management will be taken over by the Clinical Trials Unit (CTU) of the DKMS Group gGmbH based in Dresden. Among other things, the unit is responsible for providing technical support for the database and managing the resources within the Biobank. For research groups making requests, the CTU is responsible for the coordination and release of samples and/or data from the CoBi. Direct access to the samples and electronic data records is, however, excluded for the DKMS Group gGmbH.
Transplantation Centres
As equal collaborative partners of the DKMS Group gGmbH, the Transplantation Centres are responsible for the inclusion of patients and related donors. Apart from the input and administration of personal data, the Transplantation Centres are responsible for the collection and the dispatch of samples to the CoBi sample manager. They are obliged to transmit the data records and samples to the CoBi in encrypted form only.
The following Transplantation Centres are collaborative partners of the CoBi:
- University Hospital Dresden
- University Hospital Frankfurt am Main
- University Hospital Mannheim
- University Hospital Muenster
- Hospital Nuernberg
- University Hospital Leipzig
- University Hospital Bonn
- University Hospital Essen
- University Hospital Schleswig-Holstein, Kiel
- Hospital Chemnitz
- University Hospital Halle (Saale)
Collection Centres
The Collection Centres are also integrated within the CoBi as collaboration partners of the DKMS Group gGmbH. They are responsible for the inclusion of interested non-related donors and, in this context, for the collection and dispatch of the samples to the CoBi. Collection Centres are also responsible for ensuring that the data records and samples of donors are only transmitted in coded form to the CoBi.
The following Collection Centres are collaborative partners of the CoBi:
- University Hospital Dresden
- DKMS Collection Center gGmbH Dresden
- DKMS Collection Center gGmbH Cologne
- University Hospital Tuebingen
- Robert Bosch Hospital Stuttgart
- Hospital Nuernberg
- German Red Cross Blood Donor Service Baden-Wuerttemberg-Hessen gGmbH, Frankfurt am Main
- German Red Cross Blood Donor Service Nord-Ost gGmbH, Berlin
- Charité – Universitaetsmedizin Berlin
- University Hospital Schleswig-Holstein, Kiel
Scientific Committee
The Scientific Committee monitors the use of samples and/or data from the CoBi. Its tasks include the medical and scientific review and evaluation of DKMS Group gGmbH research projects that are to be carried out with the resources of the biobank.
The Scientific Advisory Board consists of the members of the Transplantation Centres and the DKMS Group gGmbH and is supported by two expert groups in the fields of immunogenetics and statistics.
Trusted Third Party and Sample Administrators
Another collaboration partner of the CoBi is the Trusted Third Party of the University Medicine in Greifswald. The Trusted Third Party is responsible for the administration of the personal identifying data and is responsible for the creation of a unique pseudonym for each participant of the Biobank. In this way, protection of the data of each participant can be ensured.
The processing and long-term storage of the samples is the responsibility of the contractually bound sample administrators. The DKMS Life Science Lab gGmbH and the DKMS Stem Cell Bank gGmbH were selected as sample administrators based on their experience in handling sensitive data and samples and a mature quality assurance concept. All tasks are coordinated and approved by the CoBi team.
A list of all essential collaboration partners can be found here.